What Best Describes the Electron Cloud Model
The electron cloud model uses the concept of orbitals referring to regions in the extra-nuclear space of an atom where electrons are likely to be found. Location where an electron has the highest probability of being found.

Atomic Orbitals Scientific Illustration Biology Atom
The electron model dictates that the electrons have no fixed position so it traces their path.

. Which statement best describes the electron cloud. The electron cloud model describes the _____ of electrons in an atom. The electron cloud model is a visual representation of the possible locations of electrons in an atom.
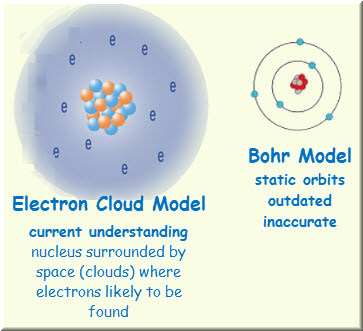
An electron cloud is an atomic model in which the atom consists of a small nucleus surrounded by a cloud of fast. It is used to describe where electrons are when they go around the nucleus of an atom. In this model of the hydrogen atom you have a black screen and then you draw a.
C It is a region in space in which the. PLUM PUDDING MODEL OF THE ATOM D. The cloud model treats the energy levels as probability clouds ie.
An orbital is a mathematical function that describes the wave-like behavior of electrons in an atom. If an oxygen atom has 8 protons and 10 neutrons in its nucleus what is its atomic number. Which best describes the electron cloud.
The electron cloud model describes which of the following. The area around the nucleus of an atom where electrons are likely to be found. Regions in which electrons are likely to be found.
The Electron Cloud Model Of An Atom Till Lindemann Metal Wings. Answer choices location where an electron has highest probability of being the group of protons that are in the nucleus the neutron orbital the negative charge of the electron Question 7 60 seconds Q. BILLIARD BALL MODEL OF THE ATOM C.
How the substance behaves in the presence of acid The arrangement of the valence electrons for oxygen and hydrogen when they bond show that the Lewis dot structure represents A bent shape Metallic bonding allows relatively free movement of electrons between atoms. The electron cloud model describes which of the following. An cloud atom model of electron the.
The cloud is darkest at the nucleus and lighter farther away representing that electrons are more likely to be found closer to the nucleus than away from it. ELECTRON CLOUD MODEL OF ATOM 2 See answers Answer 50 5 7 iandinglasagmailcom Answer. The negative charge of the electron.
Which of the following best describes the electron cloud model. Based on quantum theory which states that all matter has properties associated with a wave function the Electron Cloud Model differs from the Bohr Model in that it does not define the exact path. It shows that electrons move quickly in circular orbits.
These areas are specified by orbitals. Atomic number is equivalent to proton number. It is known that electrons are found on orbitals around the nucleus and.
Bohrs model treats electron energy levels as clearly defined orbital paths around the nucleus ike planets orbit the Sun. B It is a region in space that has a precise shape and is completely filled hva dense electron cloud. It is known that electrons are found on orbitals around the nucleus and.
Berdasarkan model atom berikut yang merupakan model atom dalton adalah. The orbitals are specified by shells and sub-orbitals. When a neutrally charged atom loses an electron to another atom the result is the creation of.
The densest area of the cloud is where the electrons have the greatest chances of being. Which statement about the electron-cloud model is true. This bonding results in the malleability of substances.
When an atom of lithium loses an electron the atom becomes a. Electron cloud is an informal way to describe an atomic orbital. The electron cloud model is a visual representation of the possible locations of electrons in an atom.
Answered Which of the following best describes the modern atomic theory. It shows that electrons remain in high-energy subshells. The electron cloud shows the.
Named Richard Feynman. The electron cloud model differs from the more simplistic Bohr model in which electrons orbit the nucleus in much the same way as planets orbit the sun. An electron cloud represents the area around an atoms nucleus where electrons are most likely to be found.
Thats because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus like the ones shown in the Figure below for a helium atom. Liquid F2 has weak dispersion force attractions between its molecules whereas liquid HF has both weak dispersion force attractions and hydrogen bonding interactions between its molecules. With the help of this function the probability of finding an electron in a given.
1 The trails left by an electron as it moves around the nucleus. Intersects With Rectangular Pools. The electron cloud model is different from the older Bohr atomic model by Niels Bohr.
SOLAR SYSTEM MODEL OF THE ATOM B. Electron cloud is an informal term in physics. It shows that electrons usually carry a negative charge.
The shape not the size of an electron cloud is determined by the electrons ____. The electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas. Which statement best describes an occupied orbital according to the quantum mechanical model.
In the cloud model there are regions where an electron may likely be found but its theoretically possible for it to be located anywhere including inside the nucleus. What is the difference between an orbital and the electron cloud. SOLAR SYSTEM MODEL OF THE ATOM.
The modern model is also commonly called the electron cloud model. 3 Its mass is lowered but it is still the same element. The group of protons that are in the nucleus.
The shape not the size of an electron cloud is determined by the electrons ____. The electron cloud is used to describe the behavior of electrons and it is useful in building a model of the atom. Which statement about the electron-cloud model is true.
At 298 K and 1 atm Br2 is a liquid with a high vapor pressure and Cl2 is a gas. A spherical electron cloud surrounding an atomic nucleus would best represent. A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement.
Named Richard Feynman. It shows that the electrons within an atom do not have sharp boundaries. What is electron cloud Class 11.

3d Printing Scientists 3d Print A Bionic Mushroom To Generate Bioelectricity Https 3dprintingindustry Com News Sci 3d Printing Stuffed Mushrooms Scientist

Electron Cloud Atomic Model Ck 12 Foundation

Gps Receivers Use The Above Equation To Correct For Time Incongruousness That Results From Einstein S Theory Of S Special Relativity Fun Science Math Tutorials

Minimalistic Line Wavy Business Card Background Material Vector Business Card Business Card Texture Business Cards Vector Templates

Chapter 2 Chemistry Of Cell Proteins Peptide Bond Biochemistry Amino Acids

Hertzsprung Russell Diagram The Purposeof This Chart Is To Show The Evolution Of Different Types Of Stars D Astronomy Science Astrophysics Space And Astronomy

Electron Cloud Model Theory Examples What Is An Electron Cloud Video Lesson Transcript Study Com
What Is The Electron Cloud Model Of The Atom Quora

Down In The Cable Basement 11 Kv Feeders Head Off The Distribution Substations Which Step Power Down To 415 V Basement Feeder Melbourne Cbd

8 5a Atoms Molecules Quiz Quizizz

Nitrogen Electron Configuration Electron Configuration Nitrogen Electrons

Atom Neon 3d Model Atom Model Atom Model Project Neon Atom Model
What Is The Electron Cloud Model Of The Atom Quora

Electron Cloud Model Theory Examples What Is An Electron Cloud Video Lesson Transcript Study Com
What Is The Electron Cloud Model Of The Atom Quora
Sol Ps 2 Properties Of Matter Standards

Nitrogen Electron Configuration Electron Configuration Nitrogen Electrons


Comments
Post a Comment